X Chromosome Inactivation: A Breakthrough in Genetic Therapy
X chromosome inactivation plays a crucial role in the genetic landscape, particularly for females who possess two copies of this chromosome. This biological process ensures that only one X chromosome is active, effectively silencing the other, a phenomenon known as chromosomal silencing. Understanding how this inactivation occurs is essential for addressing genetic disorders such as Fragile X Syndrome and Rett Syndrome, which are linked to mutations on the X chromosome. Recent discoveries reveal that a Jell-O-like substance surrounding chromosomes is integral to this process, highlighting how cells manage their genetic material. With ongoing research, scientists aim to harness this knowledge to develop innovative treatments for these challenging conditions.
The phenomenon of X-inactivation, often referred to as the Lyon hypothesis, entails the silencing of one of the two X chromosomes present in female cells to balance gene dosage with males, who have only one X chromosome. This process is pivotal in the context of genetic disorders like fragile X syndrome and Rett syndrome, where mutations on the X chromosome can lead to severe developmental challenges. Researchers have identified that a gel-like material, resembling Jell-O, is crucial for maintaining chromosome organization and enabling effective silencing of the inactive X chromosome. Recently, advances in this area have shown promise for therapeutic applications, particularly in restoring functionality to mutated genes. By better understanding chromosomal dynamics, we pave the way for potential treatments aimed at alleviating the impacts of these genetic conditions.
Understanding X Chromosome Inactivation
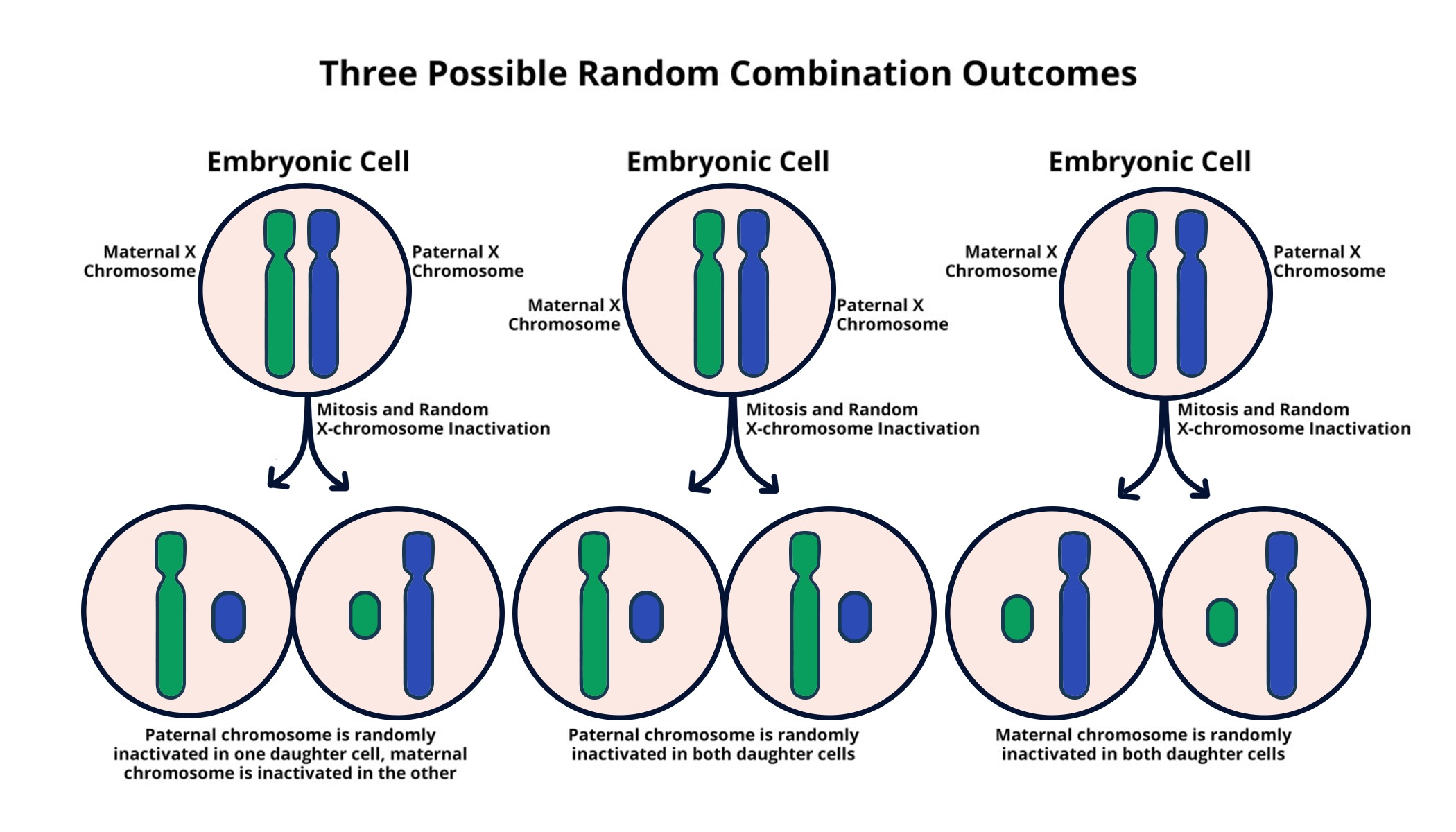
X chromosome inactivation (XCI) is a fundamental biological process that occurs in female mammals, where one of the two X chromosomes is randomly inactivated in each cell. This ensures that females, like males, have a functional dosage of the genes found on the X chromosome. The process is vital for maintaining gene balance, as excess gene dosage can lead to developmental disorders. Researchers have long sought to unravel the mechanisms behind XCI, and groundbreaking studies, such as those conducted by Jeannie T. Lee’s lab at Mass General, have shed light on the intricate molecular strategies involved in chromosomal silencing.
The inactivation process hinges on a complex interplay of RNA and chromatin modifications. One key player is the RNA product known as Xist (X-inactive specific transcript), which orchestrates the silencing of the inactivated X chromosome. Through a series of biochemical interactions, Xist alters the structure of surrounding chromatin, turning what is often described as a ‘Jell-O’ substance into a more pliable form, thus paving the way for effective gene silencing. This profound understanding of XCI could lead to innovative therapies for various genetic disorders linked to the X chromosome, including Fragile X Syndrome and Rett Syndrome.
The Role of the Jell-O Substance in Chromosomal Silencing
The jelly-like substance encasing chromosomes plays a pivotal role in chromosomal organization and function. Commonly referenced as the ‘Jell-O substance’, it acts as a protective layer, preventing chromosomes from entangling with one another. This biophysical property is crucial for the proper segregation of genetic material during cell division. Jeannie T. Lee’s research focuses on how alterations to this substance can facilitate or hinder the inactivation of the X chromosome, showcasing the delicate balance between chromosomal integrity and gene expression.
In the context of X chromosome inactivation, this Jell-O-like material interacts dynamically with Xist and other molecules involved in the silencing process. As Xist infiltrates this gelatinous layer, it modifies its characteristics, making it more amenable to the penetration of additional regulatory factors. This interaction not only aids in inactivating one X chromosome but also provides a potential target for therapies aimed at unsilencing mutated genes in patients with genetic disorders such as Fragile X and Rett syndromes. Understanding this interaction opens new avenues for treating conditions linked to X-linked mutations.
Implications for Fragile X Syndrome and Rett Syndrome
Fragile X Syndrome and Rett Syndrome are two neurodevelopmental disorders closely linked to mutations occurring on the X chromosome. Fragile X Syndrome, characterized by intellectual disabilities and developmental delays, arises from a mutation in the FMR1 gene located on the X chromosome. On the other hand, Rett Syndrome is primarily caused by mutations in the MECP2 gene and predominantly affects females. Both conditions exemplify the challenges posed by genetic disorders associated with the X chromosome, making advances in understanding X chromosome inactivation particularly significant.
The research spearheaded by Jeannie T. Lee holds promise for patients suffering from these conditions. By understanding how to leverage the mechanisms of XCI, scientists hope to develop therapeutic strategies that can effectively reactivate silenced genes where only one healthy copy exists. This therapeutic approach not only aims to provide relief for those with Fragile X and Rett syndromes but could also redefine treatment paradigms for numerous other genetic disorders linked to the X chromosome.
Chromosomal Silencing and Gene Therapy
Chromosomal silencing is a crucial mechanism regulating gene expression, particularly in polygenic disorders where gene dosage balance is critical. The insights gained from Jeannie T. Lee’s studies illuminate how chromosomal silencing can be manipulated to silence problematic mutations while allowing healthy gene expression. This understanding of gene regulation can inform future gene therapy approaches, potentially transforming how genetic disorders are treated.
The concept of targeted gene therapy rests heavily on the premise of being able to selectively reactivate silenced genes. With methodologies that can unsilence genes linked to Fragile X and Rett syndromes, researchers are laying the groundwork for innovative treatments that address the root cause of these disorders at a molecular level. This progress shows great promise for not only improving clinical outcomes but also enhancing the quality of life for those affected by such genetic conditions.
Future of X-linked Genetic Disorder Treatments
The future of treatments targeting X-linked genetic disorders like Fragile X Syndrome and Rett Syndrome is incredibly promising, thanks to recent advances in gene therapy and a deeper understanding of chromosomal mechanisms. Scientists are exploring ways to harness the principles of X chromosome inactivation and unsilencing to develop effective therapies that could significantly alter the course of these disorders. The focus is now shifting towards translating basic research into clinical applications.
As researchers continue to optimize approaches for unsilencing genes on the X chromosome, clinical trials are on the horizon, aimed at establishing the safety and efficacy of these innovative therapies. The collaborative efforts of institutions and the encouragement from funding bodies like the National Institutes of Health are accelerating this transformative journey. This could lead to not only breakthroughs in treating Fragile X and Rett Syndromes but also new strategies for various other genetic disorders linked to the X chromosome.
Molecular Mechanisms of Gene Regulation
At the molecular level, understanding gene regulation through mechanisms like X chromosome inactivation is paving the way for novel therapeutic strategies. The dynamic nature of chromatin and the interactions between various molecular players, including Xist and chromatin remodelers, highlight the complexity of gene expression regulation. As researchers delve deeper into these molecular pathways, we begin to uncover the potential for molecular therapies that can target specific genetic defects.
This intricate regulation of gene expression emphasizes the need for innovative research in genetics. By focusing on the molecular mechanisms governing chromosomal silencing, scientists are finding new approaches to rectify the gene expression profiles altered by mutations. The synergy between fundamental research and clinical insights promises to illuminate novel therapeutic avenues for patients with X-linked disorders.
Challenges and Opportunities in Genetic Research
Despite the promising advancements in understanding X chromosome inactivation, significant challenges remain in translating this knowledge from the laboratory to clinical settings. Researchers are continuously faced with hurdles, including the complexity of genetic networks, patient variability, and the need for safe delivery mechanisms for potential therapies. Achieving effective gene therapy remains a lofty goal that requires meticulous planning and research.
However, these challenges also present unique opportunities for innovation and collaboration among researchers, clinicians, and biotechnologists. The potential to develop targeted therapies for Fragile X Syndrome and Rett Syndrome not only inspires scientific inquiry but also drives forward the collective ambition to address unmet medical needs. The intersection of technological advancements and genetic research could lead to groundbreaking solutions that improve lives affected by genetic disorders.
Expanding Knowledge of Chromosomal Disorders
As research continues to expand our knowledge of X-linked disorders and the mechanisms underpinning chromosomal silencing, the implications are vast. There is a growing recognition of the importance of understanding how chromosomal behavior influences genetic disease. This holistic understanding is crucial for unraveling the complexities of various genetic disorders that extend beyond purely X-linked conditions.
By studying the nuances of X chromosome inactivation, scientists can glean insights into multifaceted genetic conditions. The lessons learned from Fragile X and Rett syndromes can inform broader genetic research, potentially revealing common pathways and therapeutic strategies applicable to a variety of disorders. The interconnectedness of these discoveries has the potential to change the landscape of genetic medicine.
The Role of Xist in X Chromosome Inactivation
Xist’s pivotal role in X chromosome inactivation cannot be overstated. This non-coding RNA is at the heart of the process that determines which X chromosome is silenced in female cells. Understanding how Xist initiates chromosomal silencing has profound implications for therapeutic interventions in conditions linked to the X chromosome. By manipulating or enhancing Xist’s function, researchers hope to develop strategies for preventing or reversing the course of X-linked diseases.
Moreover, the study of Xist provides an excellent case study for broader inquiries into RNA’s role in gene regulation. As scientists uncover the mechanisms by which Xist interacts with the Jell-O substance and other molecular components, they open new frontiers in research that may extend beyond just X-linked genetic disorders. The principles learned from X chromosome inactivation may be applicable to other chromosomal processes, including those involved in cancer and other genetic conditions.
Frequently Asked Questions
What is X chromosome inactivation and how does it affect genetic disorders?
X chromosome inactivation is a biological process in which one of the two X chromosomes in female mammals is silenced to ensure that cells have a single functional copy of the X chromosome, similar to males. This process is crucial for preventing the overexpression of X-linked genes, which can lead to genetic disorders such as Fragile X Syndrome and Rett Syndrome. Understanding X chromosome inactivation could lead to new treatments for these conditions by enabling the unsilencing of healthy genes.
How does the Jell-O-like substance relate to X chromosome inactivation?
The Jell-O-like substance refers to a gelatinous material that coats chromosomes and facilitates X chromosome inactivation. This substance creates discrete bubbles that prevent chromosomal tangling. In the context of X inactivation, an RNA molecule called Xist interacts with this Jell-O, altering its properties to facilitate the silencing of one X chromosome in females, which is essential for normal gene dosage.
Can X chromosome inactivation impact the treatment of Fragile X Syndrome?
Yes, X chromosome inactivation can significantly influence the treatment of Fragile X Syndrome. Since this disorder is often caused by mutations on the X chromosome, activating the silenced copy of the gene involved in Fragile X could provide a therapeutic benefit. Researchers, like those in Jeannie T. Lee’s lab, are exploring methods to unsilence the inactive X chromosome, potentially restoring function and alleviating symptoms.
What role do mutations play in X chromosome inactivation and genetic disorders?
Mutations on the X chromosome can lead to genetic disorders such as Fragile X Syndrome and Rett Syndrome. In cases where a mutation is present, the healthy gene on the inactivated X chromosome cannot be expressed. Researchers are investigating ways to strategically unsilence these inactivated genes through manipulation of the X chromosome’s silencing mechanisms, which could lead to effective treatments for these conditions.
How does X chromosome inactivation differ between males and females?
In females, X chromosome inactivation involves silencing one of the two X chromosomes to achieve balanced gene expression similar to that of males, who only have one X chromosome. This unique mechanism helps prevent an imbalance of X-linked genes, which is particularly relevant in conditions like Fragile X Syndrome and Rett Syndrome, where mutations can disrupt normal gene function.
What breakthroughs have been made in understanding X chromosome inactivation?
Recent breakthroughs in understanding X chromosome inactivation, particularly by Jeannie T. Lee’s lab, reveal the crucial role of Xist RNA and the Jell-O-like substance surrounding the chromosomes. These studies have provided insights into how Xist modifies the biophysical properties of the chromosomal coating, facilitating the silencing process. Such findings hold therapeutic potential for treating conditions linked to X-linked genetic disorders.
| Key Points | Details |
|---|---|
| X Chromosome Challenge | Females have two X chromosomes, while males have one, leading to the necessity for X chromosome inactivation in females. |
| Role of Xist | The X chromosome produces an RNA molecule called Xist that plays a crucial role in the inactivation process. |
| Biophysical Changes | Xist alters the properties of a gelatinous substance surrounding chromosomes, making it more pliable and enabling effective inactivation. |
| Potential Treatments | Research from Lee’s lab suggests that unsilencing inactivated X chromosomes could lead to treatments for Fragile X Syndrome and Rett Syndrome. |
| Clinical Trials | Ongoing studies aim to optimize treatments and move them into clinical trials to benefit individuals with X-linked genetic disorders. |
| Minimal Side Effects | The method may restore function of mutated genes without affecting healthy genes, suggesting low side effects. |
| Historical Context | Decades of research at Lee’s lab has led to breakthroughs despite initial focus on basic questions of X-inactivation. |
Summary
X chromosome inactivation is a crucial process that allows female cells to manage their two X chromosomes efficiently by silencing one. The recent research led by Jeannie T. Lee highlights significant advancements in understanding this complex biological mechanism. By utilizing the RNA molecule Xist, cells can effectively modify the surrounding structure to achieve inactivation. This breakthrough opens pathways for developing targeted therapies for genetic disorders linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome. As researchers continue to explore the therapeutic potential of this knowledge, the implications for future treatments could greatly improve the quality of life for those affected by these conditions.

